
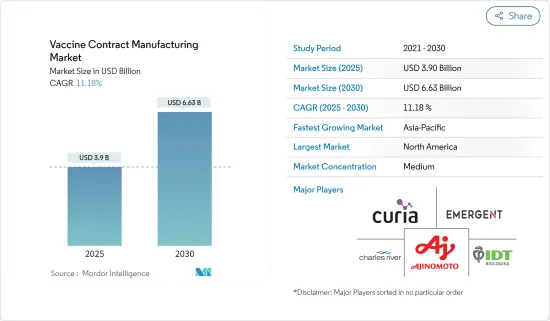
백신 수탁 제조 시장 규모는 2025년에 39억 달러, 2030년에는 66억 3,000만 달러에 달할 것으로 예측됩니다. 예측 기간(2025-2030년)의 CAGR은 11.18%를 나타낼 것으로 예상됩니다.

COVID-19 팬데믹은 백신 수탁 제조 시장에 큰 영향을 미쳤습니다. 유행성 발병 중 봉쇄는 모든 질병의 백신이 부족하여 백신 수탁 제조 시장의 성장을 억제했습니다. 그러나 안전하고 효과적인 백신을 제공하기 위해 전 세계적으로 연구개발과 임상시험이 가속화되고 백신 수요와 제조가 증가하여 궁극적으로 시장 성장을 가속했습니다. 백신 수요 증가는 제조 및 임상시험 인프라에 대한 투자 요구 증가로 이어졌습니다. 예를 들어, 유엔 국제 아동 긴급 기금(UNICEF)이 2021년 10월에 발표한 보도 자료에 따르면 새로운 ACT-Accelerator 전략이 계획되어 세계에서 COVID-19 진단, 예방 접종 및 치료법의 가용성 격차를 해결하기 위해 234억 달러의 국제 투자가 요구되었습니다. 제조규제가 해제된 이후 시장은 회복되고 있습니다. 백신 수탁 제조 시장은 예측 기간 동안 안정적인 성장률을 나타낼 것으로 예상됩니다.
또한 기술의 진보와 인프라 및 운용면에서의 비용 이점, 백신 접종을 지지하는 이니셔티브 증가, 양호한 환자층, 신생아와 소아의 백신 접종 증가 등이 조사 대상 시장의 성장에 적극적으로 영향을 미치고 있습니다.
예방접종을 모든 사람들에게 제공하는 데 중점을 둔 세계 여러 정부 및 보건기구의 이니셔티브가 예측 기간 동안 백신 수탁 제조 시장의 성장을 가속하고 있습니다. 예를 들어, 세계보건기구(WHO)에 따르면, 2022년 2월 지부티 보건부는 WHO와 유니세프의 기술 지원을 받았으며 지부티에서 약 15만 명의 어린이에게 백신을 접종하는 5일간의 전국 폴리오 백신 캠페인을 시작했습니다. 다양한 질병의 예방 대책에 초점을 맞춘 이러한 백신 추진 활동은 예측 기간 동안 시장 성장을 견인한다고 생각됩니다.
또한, 백신 기술의 기술적 진보는 유전 공학, 백신 전달 기술 및 단백질체학의 도입에 의해 촉진됩니다. 현재, 그것은 신제품의 진입으로 이어지고 있습니다. 예를 들어 2021년 3월 미국 의과대학협회(AAMC)가 발표한 논문에 따르면 mRNA 기술은 미래의 백신과 암 및 감염증 치료에 혁명을 가져올 것으로 기대되고 있습니다. 연구자들은 mRNA를 사용하면 기존 방법보다 짧고 저렴한 비용으로 다양한 백신과 치료법을 개발할 수 있다고 주장합니다. 따라서, mRNA와 같은 신기술을 백신 생산에 이용할 수 있는 이점은 시장 성장을 가속할 것으로 예상됩니다.
또한 사업 확대, 제휴, 인수 등 주요 기업이 채택한 전략적 이니셔티브 증가가 시장 성장을 가속할 것으로 추정됩니다. 예를 들어, 2022년 3월, Bharat Biotech는 새로운 결핵 백신의 개발, 제조 및 판매를 위해 스페인 바이오 제약 회사 Biofabri와 파트너십을 체결했습니다. 이 새로운 결핵 백신 MTBVAC는 사라고사 대학, 국제 에이즈 백신 이니셔티브(IAVI), 결핵 백신 이니셔티브(TBVI)와의 긴밀한 협력하에 바이오 패브리 사에 의해 제조 및 개발되고 있습니다. 이러한 시장 개척도 조사 기간 중 백신 수탁 제조 시장의 성장을 뒷받침하고 있습니다.
세계에서 백신 수탁 제조의 필요성이 높아지고 있는 것은 조사 대상 시장의 성장을 가속할 것으로 예상됩니다. 그러나 백신 비용 상승과 저장 인프라 부족은 예측 기간 동안 시장 성장을 방해할 가능성이 높습니다.
비활성화 백신에는 복제를 방지하기 위해 사멸되거나 조작된 박테리아와 바이러스가 통째로 포함되어 있습니다. 이 백신에는 살아있는 세균이나 바이러스가 포함되어 있지 않기 때문에 면역력이 매우 저하된 사람이라도 예방을 목적으로 하는 질병을 전파시킬 수 없습니다.
이것은 이런 유형의 백신의 큰 장점 중 하나입니다. 이 백신은 생백신만큼 강한 면역을 생산하거나 부여할 수 없습니다. 따라서, 더 나은 면역 반응을 얻기 위해서는 비활성화 백신을 장기간에 걸쳐 여러 번 접종해야 합니다. 대상 질환 부담의 크기, 예방 조치로서의 이러한 백신의 정기적인 수요, COVID-19 팬데믹의 출현은 예측 기간 동안 이 분야의 성장에 큰 영향을 미칠 것으로 예상됩니다.
게다가 기업뿐만 아니라 학술계의 연구자도 다양한 적응증의 비활성화 백신 후보의 연구개발에 적극적으로 임하고 있으며, 그 중에는 보건당국의 승인을 얻고 있는 것도 있습니다. 따라서, 이러한 요인들에 의해 비활성화 백신 분절이 성장할 것으로 예상됩니다. 예를 들어, 2022년 4월 현재, 세계보건기구는 COVID-19에 대한 10가지의 백신을 승인하고 있으며, 그 중 3유형은 비활성화 유형으로 CoronaVac(Sinovac), Sinopharm, COVAXIN(Bharat Biotech)을 포함합니다.
또한 2022년 4월 인도의 중앙 의약품 표준 관리기구(CDSCO)는 Bharat Biotech의 COVAXIN에 6-12세 어린이에게 긴급 사용 승인을 받았습니다. 이와 같이 비활성화 백신의 제조에 관한 연구개발의 고조와 종래의 백신 치료에 대한 비활성화 백신의 우위성이 예측 기간에 있어서의 백신 수탁 제조 시장의 성장에 영향을 미칠 것으로 예상됩니다.
백신 제조에 있어서 고도로 선진적인 기술과 시스템의 채용이 증가하고, 백신의 연구 개발에 있어서의 기술 진보가 북미 시장 성장을 밀어올릴 것으로 예상됩니다. 이 지역에서는 백신이 시장에서 이용 가능하다는 사람들의 높은 인식이 시장의 높은 성장에 기여하고 있습니다.
미국 소아과학회가 2022년 3월에 발표한 데이터에 따르면, 미국에서는 100만명 이상이 장기적으로 B형 간염에 감염되고 있으며, 아기 시절에 B형 간염에 감염된 사람은 평생 90%의 확률로 간암 등 심각한 만성 질환을 발병한다고 합니다. 그러므로 B형 간염 증가는 백신과 그 생산 수요를 증가시키고 시장 성장을 가속할 것으로 예상됩니다.
게다가 2022년 7월 WHO 업데이트에 따르면 미국은 세계 소아마비 박멸 이니셔티브의 파트너 및 2위 기증자로, 또한 개발도상국의 노력의 지원자로서 소아마비 박멸 활동에 관여하고 있습니다. 미국의 2022년 소아마비는 2억 5,300만 달러로 추정됩니다. 게다가 2021년 1월, 미국 보건 사회 복지성(HHS)은 18-26세의 젊은 성인의 인유두종 바이러스(HPV) 백신 접종율을 높이기 위해, HPV VAX NOW 캠페인을 개시했습니다. 이와 같이 백신 수탁 제조의 필요성 증가는 의료 부문 투자 증가와 함께 이 지역 시장 성장을 가속할 가능성이 높습니다.
예방 접종의 계몽을 위해 이루어진 정부의 이니셔티브도 예측 기간 동안 시장 성장을 가속합니다. 예를 들어, 2022년 8월 캐나다 정부는 캐나다에서 계절성 독감 백신 사용에 대한 최신 권장사항을 제공하는 '독감에 대한 캐나다 예방접종 가이드 장'과 '2022-2023년 계절성 독감 백신에 대한 성명'을 발표했습니다. 예를 들어, 정부는 캐나다의 18세 이상의 성인들에게 재조합 4가 계절성 독감 백신인 Supemtek(RIV4)의 사용을 허용했습니다. 이러한 이니셔티브는 이 지역에서의 예방접종에 유리하며, 예측 기간 동안 시장 성장을 가속합니다.
백신 수탁 제조 시장은 세분화된 경쟁 시장이며, 여러 대형 기업으로 구성되어 있습니다. 시장 점유율 측면에서 현재 여러 회사의 대기업이 시장을 독점하고 있습니다. 현재 시장을 독점하고 있는 기업으로는 Ajinomoto Bio-Pharma Services, Curia Global, Catalent, Charles River Laboratories International Inc., Emergent BioSolutions Inc., CJ CheilJedang Corporation(Batavia Biosciences), Gedeon Richter(Richter-Helm BioLogics, Fujifilm Holdings, AB 등이 있습니다.
The Vaccine Contract Manufacturing Market size is estimated at USD 3.90 billion in 2025, and is expected to reach USD 6.63 billion by 2030, at a CAGR of 11.18% during the forecast period (2025-2030).
The COVID-19 pandemic had a profound impact on the vaccine contract manufacturing market. During the outbreak of the pandemic, there was a shortage of vaccines for all diseases due to the lockdown, which hampered the growth of the vaccine contract manufacturing market. However, R&D and clinical trials across the world accelerated to provide a safe and effective vaccine, thus increasing the demand and manufacturing of vaccines, eventually driving the growth of the market. Increased demand for vaccines resulted in increased investment needs for the manufacturing and clinical trial infrastructure. For instance, as per a press release by the United Nations International Children's Emergency Fund (UNICEF) in October 2021, a new ACT-Accelerator strategy was planned, which called for a USD 23.4 billion international investment to address disparities in the availability of COVID-19 diagnostics, vaccinations, and therapies worldwide. The market has recovered since the manufacturing restrictions were lifted. The vaccine contract manufacturing market is expected to show a stable growth rate during the forecast period.
In addition, the advancements in technology and cost benefits in infrastructure and operational benefits, increase in initiatives favoring vaccinations, and favorable patient demographics, and growing vaccinations of newborns and children are actively affecting the growth of the market studied.
The initiatives taken by various governments and health organizations all over the world focusing on providing vaccinations to all people are driving the growth of the vaccine contract manufacturing market in the forecast period. For instance, as per the World Health Organization, in February 2022, Djibouti's Ministry of Health, with technical support from WHO and UNICEF, launched a five-day national polio vaccination campaign to vaccinate approximately 150,000 children in Djibouti. These vaccine drives focusing on preventive measures for various diseases are likely to drive the growth of the market in the forecast period.
Furthermore, technological advancements in vaccine technology have been fueled by the introduction of genetic engineering, vaccine-delivering technology, and proteomics. Currently, it is resulting in the entry of new products. For instance, according to the article published by the Association of American Medical Colleges (AAMC) in March 2021, mRNA technology promises to revolutionize future vaccines and treatments for cancer and infectious diseases. Researchers claim that mRNA can be used to create a variety of vaccines and treatments in less time and at lower costs than traditional methods. Thus, the advantage of new technology like mRNA to create vaccines is expected to drive the market's growth.
Additionally, rising strategic initiatives adopted by key players, such as business expansion, partnerships, and acquisitions, are estimated to propel the market's growth. For instance, in March 2022, Bharat Biotech entered into a partnership with the Spanish biopharmaceutical firm, Biofabri for the development, manufacturing, and marketing of a new tuberculosis vaccine. The new TB vaccine, MTBVAC, is being manufactured and developed by Biofabri in close collaboration with the University of Zaragoza, the International AIDS Vaccine Initiative (IAVI), and the Tuberculosis Vaccine Initiative (TBVI). Such developments by market players are also boosting the growth of the vaccine contract manufacturing market in the study period.
The increase in the necessity of vaccine contract manufacturing around the world is expected to propel the growth of the market studied. However, the increasing cost of vaccines, along with the lack of storage infrastructure, is likely to hinder the growth of the market in the forecast period.
The inactivated vaccines contain whole bacteria or viruses that have been killed or manipulated to prevent replication. As these vaccines do not contain any live bacteria or viruses, they cannot spread the diseases they are intended to prevent, even in those with highly compromised immune systems, which is one of the major advantages of this type of vaccine.
These vaccines do not produce or confer immunity as strong as live-attenuated vaccines. Hence, several doses of inactivated vaccines are required over time for better immune response. The high burden of the target diseases, regular demand for these vaccines as a preventive measure, and the emergence of the COVID-19 pandemic are expected to significantly impact the segment's growth over the forecast period.
In addition, the companies, as well as researchers from academics, are actively involved in the research and development of inactivated vaccine candidates for different indications, some of which are even approved by the health authorities. Hence, due to these factors, the inactivated vaccine segment is expected to grow. For instance, as of April 2022, the World Health Organization has approved ten vaccines against COVID-19, of which three are inactivated types and include CoronaVac (Sinovac), Sinopharm, and COVAXIN (Bharat Biotech).
Furthermore, in April 2022, the Central Drugs Standard Control Organization (CDSCO) of India provided emergency use approval to Bharat Biotech's COVAXIN for children between 6-12 years of age. Thus, the rise in the research and development of the production of inactivated vaccines and the advantages of inactivated vaccines over conventional vaccine treatments are expected to influence the growth of the vaccine contract manufacturing market in the forecast period.
The rise in the adoption of highly advanced techniques and systems in vaccine manufacturing and the technological advancements made in vaccine research and development studies is expected to boost the growth of the market in the North American region. The high awareness among the population about the availability of vaccines in the market contributes to the high market growth in the region.
According to data published by the American Academy of Pediatrics in March 2022, more than 1 million people in the United States have long-term hepatitis B infections, and people who are infected with hepatitis B as a baby have a 90% chance of developing serious, chronic conditions like liver cancer in their lifetime. Thus, an increase in hepatitis B is expected to increase the demand for vaccines and their manufacturing, thereby driving the growth of the market.
Additionally, according to the WHO Updates in July 2022, the United States has been involved in polio-eradication efforts, both as a partner and the second-largest donor to the Global Polio Eradication Initiative and as a supporter of developing-country efforts. Polio funding in the United States is estimated to have been USD 253 million in 2022. Furthermore, in January 2021, the United States Department of Health and Human Services (HHS) launched the HPV VAX NOW campaign to increase human papillomavirus (HPV) vaccination rates among young adults aged 18-26. Thus, the increasing necessity for vaccine contract manufacturing coupled with increasing investment in the health care department is likely to propel the growth of the market in this region.
The government initiatives undertaken for vaccination awareness also propel the market's growth during the forecast period. For instance, in August 2022, the Government of Canada published the Canadian Immunization Guide Chapter on Influenza and Statement on Seasonal Influenza Vaccine for 2022-2023, which provides updated recommendations regarding the use of seasonal influenza vaccines in Canada. For instance, the government authorized the use of Supemtek (RIV4), a recombinant quadrivalent seasonal influenza vaccine, for use in Canadian adults of age 18 and older. Such initiatives favor vaccination in the region and thereby boost the growth of the market during the forecast period.
The vaccine contract manufacturing market is fragmented and competitive and consists of several major players. In terms of market share, a few of the major players are currently dominating the market. Some companies which are currently dominating the market are Ajinomoto Bio-Pharma Services, Curia Global, Catalent, Charles River Laboratories International Inc., Emergent BioSolutions Inc., CJ CheilJedang Corporation (Batavia Biosciences), Gedeon Richter (Richter-Helm BioLogics, Fujifilm Holdings Corporation, ICON PLC, IDT Biologika GmbH, Lonza Group AG, and Recipharm AB, among others.