
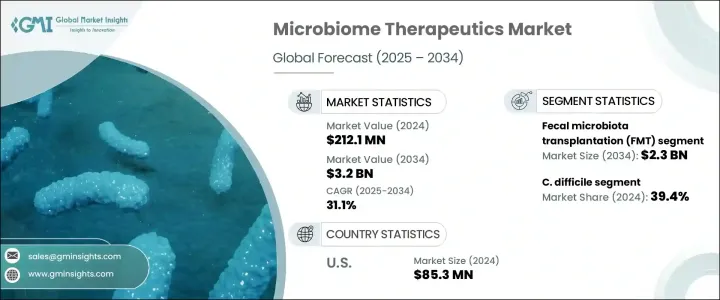
세계의 마이크로바이옴 치료제 시장은 2024년 2억 1,210만 달러로 평가되었고, CAGR 31.1%로 성장하여 2034년까지 32억 달러에 이를 것으로 추정됩니다.
이 분야의 현저한 성장은 연구개발에 대한 투자 증가, 선진적인 의약품 개발을 목표로 한 파트너십 증가, 맞춤형 의료에 대한 폭넓은 뒷받침에 의한 점이 큽니다. 미생물의 생태계를 보다 깊게 통찰할 수 있는 개발 기술에 의해 보다 정밀한 치료법의 개발이 가능하게 되어, 그 결과, 진단 능력이 향상해, 치료법도 세련되어 왔습니다.

마이크로바이옴 치료제 인공 미생물, 살아있는 바이오 치료제, 마이크로바이옴 조정 화합물을 이용하여 다양한 만성 질환에 대처합니다. 치료법에 대한 적용은 면역조절, 위장의 건강, 대사의 불균형, 암 관련 질환 등에 이르렀습니다.
| 시장 범위 | |
|---|---|
| 시작 연도 | 2024년 |
| 예측 연도 | 2025-2034년 |
| 시작 금액 | 2억 1,210만 달러 |
| 예측 금액 | 32억 달러 |
| CAGR | 31.1% |
시장을 유형별로 분류하면 분변 미생물총 이식(FMT)과 마이크로바이옴 의약품으로 분류됩니다. FMT는 특히 기존의 항생제에서는 효과가 지속되지 않는 지속성 위장 감염증의 치료에 있어서 그 효능이 널리 인정되고 있습니다.
용도의 관점에서 시장은 염증성 장 질환(IBD), 암, 당뇨병, 클로스트리디오이데스 디피실(C. 디피실), 기타 적응증으로 구분됩니다. 클로스트리디오이데스 디피실 분야는 2024년에 39.4%의 시장 점유율을 획득하여 최고 공헌도로 부상했습니다. 클로스트리디오이데스 디피실 감염의 중증화는 종종 대장염과 패혈증과 같은 심각한 건강 합병증을 일으키며 건강 관리 제공업체에게 큰 관심사가 되고 있습니다. 기존의 항생제 치료에 따른 높은 재발률(25%에서 30%로 추정)을 고려하면 보다 효과적이고 지속 가능한 해결책이 급무입니다.
지역별로는 미국이 2024년 매출액으로 8,530만 달러를 차지하고 있습니다. 30.1%를 보일 것으로 예측됩니다. 위장 장애와 대사 장애 증가가 이 성장에 크게 기여하고 있습니다.
마이크로바이옴 의약품 개발에서 견고한 파이프라인을 갖춘 신흥 바이오테크놀러지 기업의 존재도 미국 시장의 주요 성장 요인 중 하나입니다. 규제 당국의 승인도 합리화되고 있으며, 기업은 최첨단 솔루션을 보다 효율적으로 도입할 수 있게 되어 있습니다.
The Global Microbiome Therapeutics Market was valued at USD 212.1 million in 2024 and is estimated to grow at a CAGR of 31.1% to reach USD 3.2 billion by 2034. The remarkable growth in this field is largely driven by heightened investment in research and development, increased partnerships targeting advanced drug development, and a broader push toward personalized medicine. Favorable regulatory pathways, including accelerated drug approval mechanisms, have also contributed to this upward trend. In addition, scientific progress in understanding the complex interactions between microbes and the human host has enhanced the development of targeted therapeutic solutions. Technologies allowing for deeper insights into microbial ecosystems are making it possible to develop more precise treatments, which, in turn, have led to better diagnostic capabilities and more refined therapies. As diagnostic methods evolve, the ability to match patients with appropriate microbiome-based solutions becomes more efficient, further boosting the demand for these therapies.
Microbiome therapeutics utilize engineered microorganisms, live biotherapeutics, and microbiome-modulating compounds to address a range of chronic conditions. These approaches include both microbiome drugs and fecal microbiota transplantation (FMT). The therapeutic applications cover immune regulation, gastrointestinal health, metabolic imbalances, and cancer-related conditions. As public awareness grows and the clinical benefits become more evident, these treatment modalities continue to see broader adoption across medical settings.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $212.1 Million |
| Forecast Value | $3.2 Billion |
| CAGR | 31.1% |
When broken down by type, the market is categorized into fecal microbiota transplantation (FMT) and microbiome drugs. In 2024, the FMT segment dominated the market by generating USD 158.3 million in revenue. This segment is projected to reach USD 2.3 billion by 2034, maintaining its leading position with an expected CAGR of 30.7% over the forecast period. FMT has earned widespread recognition for its effectiveness, particularly in treating persistent gastrointestinal infections where conventional antibiotics fail to yield lasting results. Its growing credibility in clinical practice has contributed to its increasing usage in both hospital and outpatient settings. With expanding implementation across healthcare institutions, the demand for FMT-based therapeutics continues to climb.
From an application standpoint, the market is segmented into inflammatory bowel disease (IBD), cancer, diabetes, Clostridioides difficile (C. difficile), and other indications. Among these, the C. difficile segment emerged as the top contributor, capturing a 39.4% market share in 2024. This segment is expected to grow at a CAGR of 31.6% between 2025 and 2034. The severity of C. difficile infections often leads to critical health complications such as colitis and sepsis, making it a major concern for healthcare providers. As a common hospital-acquired infection, particularly in long-term care environments, it poses a considerable burden on healthcare systems. Given the high recurrence rates associated with traditional antibiotic treatment-estimated between 25% to 30%-there is a pressing need for more effective and sustainable solutions. Microbiome-based therapies address this need by restoring gut flora and enhancing immune response, which positions them as both cost-effective and clinically valuable alternatives.
In the regional landscape, the United States accounted for USD 85.3 million in microbiome therapeutics revenue in 2024. The US market is forecast to grow at a CAGR of 30.1% from 2025 to 2034. Rising incidences of gastrointestinal and metabolic disorders have significantly contributed to this growth. Infections such as C. difficile remain prevalent, particularly among individuals with compromised immune systems. As a result, demand continues to rise for innovative therapeutic options that can address these issues more effectively than traditional treatments.
The presence of leading biotechnology firms with robust pipelines in microbiome drug development is another key growth driver in the US market. These companies are at the forefront of innovation, investing heavily in clinical trials and forming strategic alliances to bring novel therapies to market. Regulatory approvals are also becoming more streamlined, further enabling companies to introduce cutting-edge solutions with greater efficiency. With a highly competitive landscape marked by continuous advancements and collaborations, the microbiome therapeutics market is poised for sustained expansion in the years ahead.