
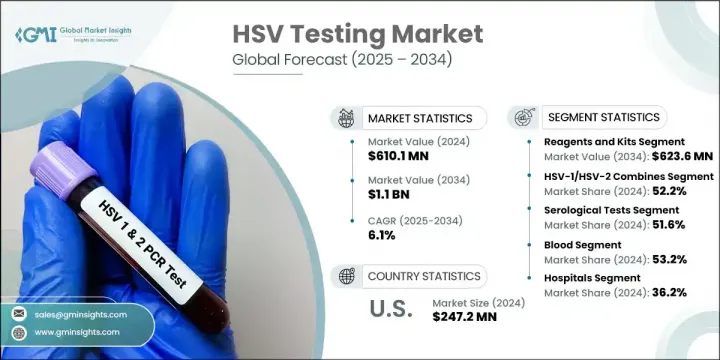
세계의 HSV 검사 시장은 2024년에 6억 1,010만 달러로 평가되었으며 CAGR 6.1%로 성장할 전망이며 2034년까지는 11억 달러에 이를 것으로 추정됩니다.
이러한 상승 추세는 주로 단순 포진 바이러스 감염의 전 세계적 유병률 증가와 함께 성매개감염(STI)에 대한 인식 제고, 그리고 조기 및 정확한 진단의 중요성에 기인합니다. 임상 및 가정용 검사 솔루션에 대한 수요 증가가 시장 역학을 지속적으로 형성하고 있습니다. 다중 PCR, 자동화된 혈청학적 플랫폼, 신속 검사 키트, 현장 진단(POCT)와 같은 진단 기술 혁신은 특히 의료 서비스가 부족한 지역과 대량 처리 환경에서 정확성과 접근성 향상을 촉진하고 있습니다.

성 건강 증진 프로그램의 확산, 강화된 선별 정책, 그리고 HSV 검사의 다중 질환 패널 및 전자 건강 시스템 통합 확대가 시장 채택을 촉진하고 있습니다. 환자 중심의 접근 가능한 진단으로의 전반적인 전환은 의료 부문이 신속하고 분산된 검사 방식을 점점 더 선호하도록 촉진하고 있습니다. 이러한 검사는 낙인을 줄이고, 더 빠른 임상적 결정을 지원하며, 외래 및 재택 치료 환경에서 더 큰 편의성을 제공함으로써 광범위한 공공 보건 전략에서의 가치를 강화합니다.
| 시장 범위 | |
|---|---|
| 시작 연도 | 2024년 |
| 예측 연도 | 2025-2034년 |
| 시작 금액 | 6억 1,010만 달러 |
| 예측 금액 | 11억 달러 |
| CAGR | 6.1% |
HSV-1/HSV-2 복합 검사 부문은 단일 검사로 두 유형의 단순 헤르페스 바이러스를 모두 검출할 수 있는 능력 덕분에 2024년 52.2%로 가장 큰 점유율을 기록했습니다. 분자 및 혈청학적 형식을 모두 포괄하는 이러한 복합 검사는 진단 워크플로우를 간소화하고 임상 및 공공보건 환경 전반에서 선별 정확도를 높이기 위해 설계되었습니다. 증상이 있는 감염과 무증상 감염을 모두 식별할 수 있는 능력은 특히 생식 건강 및 산전 관리 상황에서 더 나은 정보에 기반한 의료 결정을 지원합니다. 고위험군에서 효율적인 진단에 대한 수요가 증가함에 따라 이중 표적 검사는 포괄적인 성매개감염증(STI) 패널에서 표준이 되고 있습니다.
2024년 검사 유형 별 점유율에서 혈청학적 검사가 51.6%로 가장 큰 비중을 차지했습니다. 이러한 항체 기반 검사는 특히 뚜렷한 증상이 없는 환자에서 과거 HSV 노출 여부를 확인하는 데 매우 유용합니다. 대규모 선별 검사 프로그램, 산전 관리, 일반 성매개감염 진단 등 다양한 분야에서 활용됩니다. 대량 실험실 운영과의 호환성 덕분에 혈청학적 검사는 자동화된 데이터 출력, 효율적인 일괄 검사, 중앙 집중식 진단 시스템과의 원활한 통합을 제공합니다. 확장성과 비용 효율성 덕분에 전 세계 병원, 실험실 및 공공 보건 프로그램에서 필수 도구로 자리매김했습니다.
미국의 시장 상황은 2024년 2억 4,720만 달러를 기록하며 전 세계 HSV 검사 미래의 핵심 기여자로서의 역할을 공고히 했습니다. HSV 감염이 성인 인구 전반에 널리 퍼져 있어 신뢰할 수 있는 진단에 대한 수요는 여전히 높습니다. 어린 시절에 주로 감염되는 경구 헤르페스와 흔한 성병인 생식기 헤르페스 모두 국가적 검사량 증가에 기여합니다. 지원적인 의료 인프라, 적극적인 질병 인식 노력, 지속적인 연구 투자는 미국의 시장을 더욱 공고히 합니다.
세계의 HSV 검사 시장을 선도하는 주요 기업은 Abbott Laboratories, TRUPCR, Quest Diagnostics, McKesson Medical-Surgical, Bio-Rad Laboratories, Thermo Fisher Scientific, CTK Biotech, Quidel Ortho Corporation, Innermost Healthcare, DiaSorin, Hologic, Scientific, bioWORLD, Meridian Bioscience, PrivaPath Diagnostics, F. Hoffmann-La Roche, Becton, Dickinson and Company 등이 있습니다. HSV 검사 시장에서 경쟁하는 기업들은 기술 혁신, 포트폴리오 확장, 전 세계 진출에 주력하며 입지를 강화하고 있습니다. 주요 전략으로는 분산형 및 조기 검출을 지원하는 신속, 현장 진단(POCT), 복합 진단 검사 개발이 포함됩니다. 기업들은 데이터 관리 및 워크플로우 효율성 향상을 위해 진단 도구의 자동화 및 전자 건강 플랫폼과의 통합에 투자하고 있습니다. 의료 서비스 제공자, 공공보건 기관 및 유통업체와의 파트너십은 특히 서비스가 부족한 시장에서 접근성이 확대되도록 돕습니다. 정확성, 경제성 및 사용 편의성에 대한 지속적인 개선은 제품이 증가하는 공공보건 수요를 충족하도록 보장합니다.
The Global HSV Testing Market was valued at USD 610.1 million in 2024 and is estimated to grow at a CAGR of 6.1% to reach USD 1.1 billion by 2034. This upward trajectory is largely fueled by the increasing global prevalence of herpes simplex virus infections, along with heightened awareness surrounding sexually transmitted infections (STIs) and the critical importance of early and accurate diagnosis. Rising demand for both clinical and at-home testing solutions continues to shape market dynamics. Innovations in diagnostic technologies-such as multiplex PCR, automated serological platforms, rapid kits, and point-of-care testing-are driving improved accuracy and accessibility, particularly in underserved regions and high-throughput settings.
Widespread sexual health initiatives, enhanced screening policies, and the growing integration of HSV testing into multi-disease panels and electronic health systems are contributing to stronger adoption. The overall shift toward patient-centered, accessible diagnostics is also prompting the healthcare sector to increasingly prefer rapid, decentralized testing formats. These tests reduce stigma, support quicker clinical decisions, and offer more convenience in outpatient and home-care environments, reinforcing their value in broader public health strategies.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $610.1 Million |
| Forecast Value | $1.1 Billion |
| CAGR | 6.1% |
The HSV-1/HSV-2 combined testing segment held the largest share in 2024 at 52.2%, driven by its ability to detect both types of herpes simplex virus in a single test. These combination assays, covering both molecular and serological formats, are designed to streamline diagnostic workflows and enhance screening accuracy across clinical and public health environments. Their capacity to identify both symptomatic and asymptomatic infections supports better-informed medical decisions, particularly in reproductive health and prenatal care contexts. As demand rises for efficient diagnosis in high-risk groups, dual-target assays are becoming standard in comprehensive STI panels.
Serological tests also captured the largest share of 51.6% within the testing type category in 2024. These antibody-based tests are highly valued for detecting prior HSV exposure, especially in patients with no visible symptoms. Their use spans large-scale screening programs, prenatal care, and general STI diagnostics. Thanks to their compatibility with high-volume laboratory operations, serological tests offer automated data output, efficient batch testing, and smooth integration with centralized diagnostics. Their scalability and cost-effectiveness make them essential tools in hospitals, labs, and public health programs worldwide.
United States HSV Testing Market generated USD 247.2 million in 2024, cementing its role as a key contributor to the global HSV testing landscape. With HSV infections widespread across the adult population, the need for reliable diagnostics remains strong. Oral herpes, often contracted early in life, and genital herpes, a common STI, both contribute to high national testing volumes. Supportive healthcare infrastructure, active disease awareness efforts, and continued research investment further strengthen the country's market position.
Notable companies leading the Global HSV Testing Market include Abbott Laboratories, TRUPCR, Quest Diagnostics, McKesson Medical-Surgical, Bio-Rad Laboratories, Thermo Fisher Scientific, CTK Biotech, QuidelOrtho Corporation, Innermost Healthcare, DiaSorin, Hologic, AdvaCare Pharma, ZEUS Scientific, bioWORLD, Meridian Bioscience, PrivaPath Diagnostics, F. Hoffmann-La Roche, and Becton, Dickinson and Company. Companies competing in the HSV testing market are advancing their positions by focusing on technological innovation, portfolio expansion, and global outreach. A major strategy includes the development of rapid, point-of-care, and combination diagnostic tests that support decentralized and early detection. Firms are investing in automation and integration of diagnostic tools with electronic health platforms to improve data management and workflow efficiency. Partnerships with healthcare providers, public health agencies, and distributors help in expanding reach, especially in underserved markets. Continuous improvements in accuracy, affordability, and ease of use ensure that products meet growing public health demands.