
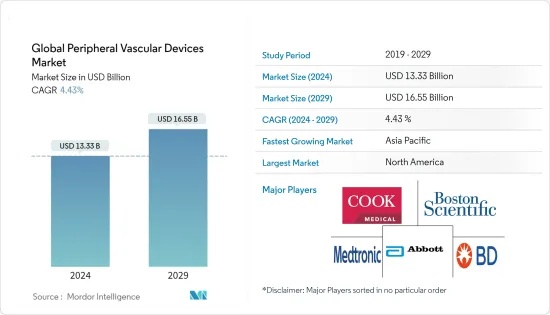
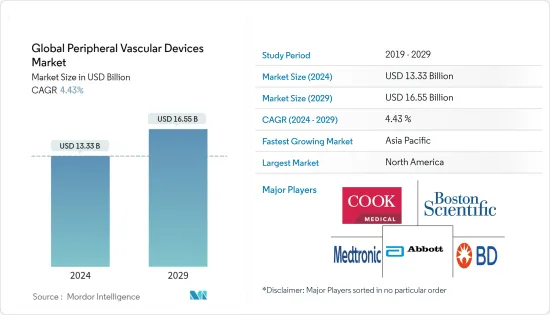
세계 말초혈관 기기 시장 규모는 2024년 133억 3,000만 달러로 추정되며, 2029년까지 165억 5,000만 달러에 달할 것으로 예상되며, 예측 기간(2024-2029년) 동안 4.43%의 CAGR로 성장할 것으로 예상됩니다.
COVID-19 팬데믹은 진단 및 치료 절차뿐만 아니라 이 분야의 연구 개발 활동에도 영향을 미쳐 COVID-19 이외의 치료 및 진단 절차에 부정적인 영향을 미쳤으며, 이는 조사 대상 시장에 큰 영향을 미쳤습니다. 또한, 많은 연구에 따르면 심장 질환을 앓고 있는 사람들이 COVID-19로 인해 큰 위험에 노출되어 있으며, 이로 인해 병원과 진단 센터에 입원하는 환자 수가 더욱 감소하고 있습니다. 예를 들어, 2021년 5월 국립생명공학정보센터가 발표한 'COVID-19가 영국의 심장 수술 활동 및 관련 30일 사망률에 미치는 영향'이라는 제목의 연구 결과에 따르면, COVID-19 팬데믹 기간 동안 영국에서 심장 수술 건수가 급격히 감소했습니다. 약 45,000건의 수술이 부족했지만, 팬데믹 기간 동안 시행된 대부분의 심장 수술은 사망 위험이 증가하지 않은 것으로 나타났습니다. 이 연구는 COVID-19가 순환기과 서비스에 부정적인 영향을 미치고 있음을 보여줍니다. 또한 이 연구는 영국에서 진행되었습니다.
그러나 COVID-19 감염자 수가 감소하고 봉쇄령이 해제되면서 시장이 활기를 띠기 시작했습니다. 예를 들어, 호주 보건 복지 연구소의 2022년 5월에 따르면, 1,180만 건의 입원 중 7.0%는 중환자실 입원이었고, 3.8%는 심혈관 질환으로 인한 입원이었습니다. 이러한 응급 및 응급실 입원의 증가는 동맥폐색 치료의 가용성에 대한 필요성을 야기하며, 이는 분석 기간 동안 조사된 말초혈관 기기 시장의 성장을 촉진할 것으로 예상됩니다.
또한, 최소침습수술에 대한 수요 증가와 말초동맥질환(PAD)의 발생률 증가가 시장 성장에 긍정적인 영향을 미치고 있습니다.
미국심장협회의 2021년 보고서에 따르면, 말초동맥질환(PAD)은 전 세계적으로 2억 명 이상이 앓고 있으며, 높은 사망률과 이환율과 관련이 있습니다. 세계 인구가 고령화됨에 따라 PAD는 미래에 더욱 보편화 될 수 있습니다. 따라서 통계에 따르면 PAD의 수가 더 빠른 속도로 증가하고 있으며 궁극적으로 말초혈관 기기 시장을 주도하고 있음을 보여줍니다.
2021년 8월 미국심장협회가 발표한 '하지 말초동맥질환: 현대의 역학, 관리 격차 및 미래 방향'이라는 제목의 과학적 연구에 따르면, 하지 말초동맥질환(PAD)은 전 세계적으로 2억 3천만 명 이상이 앓고 있으며, 관련성이 있다고 합니다. 관상동맥성 심장병, 뇌졸중과 같은 심혈관 질환, 절단 상태와 같은 사지 결과 등 여러 가지 불리한 임상 결과의 위험이 증가합니다. PAD 발생률의 증가는 결국 예측 기간 동안 말초혈관 기기 시장을 견인할 것으로 보입니다.
따라서 앞서 언급한 요인으로 인해 조사 대상 시장은 분석 기간 동안 성장할 것으로 예상됩니다. 그러나 높은 설치 및 유지보수 비용으로 인해 시장 성장을 저해할 수 있습니다.
심장 질환의 증가로 인해 전 세계적으로 말초혈관 스텐트에 대한 수요가 증가하고 있습니다. 미국 심장 협회(AHA)의 심장병 및 뇌졸중 통계 - 2022년 최신 데이터에 따르면 심혈관 질환(CVD)은 2020년에 전 세계적으로 1,905 만 명이 사망 한 주요 기본 사망 원인으로 나열되어 있습니다. 같은 자료에 따르면 2020년 전 세계적으로 약 708만 명이 뇌혈관 질환으로 사망한 것으로 추정됩니다. 이 나라 인구의 심장병 유병률이 매우 높기 때문에 말초혈관 스텐트에 대한 수요가 증가했습니다.
기술의 발전과 제품 승인 증가, 주요 기업들의 파트너십과 협업이 시장 성장에 기여하고 있습니다. 예를 들어, 세계 심혈관 기술 기업 Cordis는 2022년 3월 미국 식품의약국(FDA)의 승인을 받은 SMART RADIANZ 혈관 스텐트 시스템(SMART RADIANZ 혈관 스텐트 시스템)은 요골 말초 수술용으로 특별히 설계된 자가 확장형 스텐트입니다. 이 시스템은 최근 승인된 혈관 스텐트 시스템, BRITE TIP RADIANZ 가이딩 시스 및 SABERX RADIANZ PTA 카테터와 함께 완성됩니다. 이 시스템은 방사형 접근을 최적화하고 눈에 띄는 결과를 만들어내며 환자 만족도를 높이기 위해 특별히 제작되었습니다.
또한, 2021년 10월 보스턴 사이언티픽 코퍼레이션은 라스베가스에서 개최된 VIVA(Vascular InterVentional Advances) 컨퍼런스에서 엘루비아 약물 용출 혈관 스텐트 시스템의 우수한 임상시험 결과를 발표했습니다. 발표된 EMINENT 연구 데이터에 따르면, 엘루비아 스텐트는 최대 210mm 길이의 말초동맥질환(PAD) 및 얕은 대퇴동맥(SFA) 병변 환자 치료에서 자가 확장형 베어메탈 스텐트(BMS)보다 우수한 성능을 보였다고 합니다. 이 연구에는 775명의 환자가 참여했으며, 이는 PAD 치료에 대한 최대 규모의 약물 용출 스텐트 무작위 시험입니다. 이러한 개발은 말초혈관 스텐트의 사용을 촉진할 것으로 예상됩니다.
따라서 조사 대상 시장의 말초혈관 스텐트 부문은 성장하고 있습니다.
북미 지역은 심혈관 질환 발생률 증가, 노인 인구 증가, 이 지역에서 업계 관계자의 강력한 존재감, 의료 인프라 개선, 사용 가능한 기술에 대한 국민과 의료 산업 이해 관계자의 인식, 미국에 집중된 시장 참여자 등 다양한 요인을 가지고 있습니다.
2020년 9월에 발표된 질병통제예방센터(CDC)의 '심장병의 사실'이라는 제목의 기사에 따르면, 심장병은 미국의 주요 사망 원인으로 꼽힙니다. 또한 매년 약 80만 5,000명의 미국인이 심장마비로 사망하고 있다고 보고했습니다. 심장병으로 인한 사망자 수가 증가함에 따라 말초혈관 기기는 동맥 폐쇄 및 동맥 협착 치료에 도움이되므로 심장병에 대한 적절한 치료가 계속 필요하며 예측 기간 동안 성장할 것으로 예상됩니다.
특히 미국에서의 제품 출시, 제휴 및 인수합병이 증가하면서 시장 성장이 가속화되고 있습니다. 예를 들어, 2022년 3월 지멘스 헬시니어스(Siemens Healthineers)는 미국에서 차세대 ACUSON AcuNav Volume 4D ICE 카테터를 출시했습니다. 회사 측에 따르면 AcuNav Volume ICE 카테터는 이전에 구조적 심장 시술을 받을 수 없었던 환자들을 치료할 수 있게함으로써 의료 서비스 제공에 변화를 가져올 것이라고 합니다.
또한 2021년 9월, Abbott는 말초 혈전 제거를 위해 설계된 최소 침습적 기계식 흡입 혈전 제거 시스템을 갖춘 의료기기 회사인 Walk Vascular LLC를 인수한다고 발표했습니다. Walk Vascular의 말초 혈전 제거 시스템은 Abbott의 기존 혈관 내 제품 포트폴리오에 통합될 예정입니다. 이번 제휴를 통해 Abbott는 말초혈관 서비스 범위를 확대할 수 있게 됐습니다.
따라서 위의 요인으로 인해 조사 대상 시장은 북미 지역에서 성장할 것으로 예상됩니다.
말초혈관 기기 시장은 세계 및 지역적으로 사업을 전개하는 여러 기업이 존재하기 때문에 본질적으로 약간 통합된 시장입니다. 경쟁 상황에는 시장 점유율을 보유하고 있고 잘 알려진 몇몇 국제 및 현지 기업들에 대한 분석이 포함되어 있습니다.
The Global Peripheral Vascular Devices Market size is estimated at USD 13.33 billion in 2024, and is expected to reach USD 16.55 billion by 2029, growing at a CAGR of 4.43% during the forecast period (2024-2029).
The COVID-19 pandemic negatively affected the treatment and diagnostics procedures other than COVID-19 which had a significant impact on the studied market as it not only affected diagnostic and treatment procedures but also research and development activities in the area. Further, many studies suggested that people with cardiac diseases were at major risk from COVID-19, which further led to the reduction in footfall in hospitals and diagnostic centers. For instance, according to the research study titled 'Impact of COVID-19 on cardiac procedure activity in England and associated 30-day mortality' published by the National Center for Biotechnology Information in May 2021, during the COVID-19 pandemic, cardiac procedural activity in England decreased dramatically, with a deficit of about 45,000 procedures, with no increase in the risk of mortality for most cardiac procedures conducted during the pandemic. This study shows the negative impact of COVID-19 on cardiology services. Further, the study was conducted in England.
However the market started to gain traction as the COVID-19 cases declined and the lockdowns were taken off. For instance, according to the Australian Institute of Health and Welfare May 2022, out of 11.8 million admissions, 7.0% of hospitalizations involved a stay in the intensive care unit, and 3.8% of hospitalizations involved Cardio vascular diseases. Such increasing admission in emergency and critical care created the need for the availability of arterial blockage treatment and this is expected to drive the growth of the peripheral vascular devices market studied over the analysis period.
In addition to it, rising demand for minimally-invasive procedures and an increase in the incidence of peripheral arterial disease (PAD) are actively affecting the growth of the studied market.
According to the American Heart Association 2021 report, Peripheral artery disease (PAD) affects more than 200 million people worldwide and is associated with high mortality and morbidity. With the aging global population, it is likely that PAD may be increasingly common in the future. Hence, the statistics show that the number of PAD is increasing at a faster pace, which is ultimately driving the market for peripheral vascular devices.
A Scientific study titled 'Lower Extremity Peripheral Artery Disease: Contemporary Epidemiology, Management Gaps, and Future Directions' published by the American Heart Association in August 2021 stated that lower extremity peripheral artery disease (PAD) affects more than 230 million persons worldwide and is linked to an elevated risk of a number of unfavorable clinical outcomes (including cardiovascular diseases like coronary heart disease and stroke and limb outcomes like amputee status). The increased incidence of PAD is ultimately boost the peripheral vascular devices market over the forecast period.
Therefore, owing to the aforementioned factors the studied market is anticipated to witness growth over the analysis period. However, the high cost of installation and maintenance is likely to impede the market growth.
Due to the increase in the number of cardiac disorders there is an increased demand for peripheral vascular stents globally. The American Heart Association (AHA), Heart Disease and Stroke Statistics - 2022 Update data shows that cardiovascular disease (CVD) is listed as the primary underlying cause of death accounting for 19.05 million deaths all over the world in the year 2020. As per the same source, around 7.08 million deaths worldwide were attributed to cerebrovascular diseases in the year 2020. Such a high prevalence of cardiac disease among the population in the country reaped the demand for peripheral vascular stents.
The advancements in technology and increasing product approvals, along with partnerships and collaborations by key players are helping in the market growth. For instance, in March 2022, Cordis, a global cardiovascular technology company, announced the United States Food and Drug Administration (FDA) approval for the S.M.A.R.T. RADIANZ Vascular Stent System, a self-expanding stent specifically designed for radial peripheral procedures The RADIANZ Radial Peripheral System is completed by the recently approved vascular stent system, BRITE TIP RADIANZ Guiding Sheath, and SABERX RADIANZ PTA Catheter. This system was created specially to optimize radial access, produce remarkable results, and increase patient satisfaction.
Furthermore, in October 2021, Boston Scientific Corporation presented favorable clinical trial results for the Eluvia Drug-Eluting Vascular Stent System during a clinical trial presentation at the Vascular InterVentional Advances (VIVA) meeting in Las Vegas. The Eluvia stent outperformed self-expanding bare metal stents (BMS) for the treatment of patients with peripheral arterial disease (PAD) and superficial femoral artery (SFA) lesions up to 210 mm in length, according to data from the EMINENT trial that were presented. The study included 775 patients, making it the biggest drug-eluting stent randomized trial for PAD treatment to date. Such developments are anticipated to fuel the usage of peripheral vascular stents.
Therefore, there is a growth in the Peripheral vascular stents segment of the studied market.
North America is expected to dominate the market owing to factors such as the rising incidence of cardiovascular diseases, growing geriatric population, the strong presence of industry players in the region, better healthcare infrastructure, awareness among people and healthcare industry stakeholders about available technologies, and the high concentration of market players in the United States.
According to the Centers for Disease Control and Prevention (CDC)'s article titled 'Heart Disease Facts' published in September 2020, heart disease is the leading cause of death in the United States. The same source also reports that every year about 805,000 Americans have a heart attack. As the number of deaths due to heart diseases is increasing there is a continuous need for the proper treatment of cardiac diseases since peripheral vascular devices provides helps in the treatment of artery blockages and narrowing and hence are expected to show growth over the forecast period.
The increasing product launches, partnerships, and acquisitions particularly in the United States are leading to an increase in market growth. For instance, in March 2022, Siemens Healthineers launched next-generation ACUSON AcuNav Volume 4D ICE Catheter in the United States. As per the company, AcuNav Volume ICE catheter transforms care delivery by enabling the treatment of patients who were previously not able to undergo Structural Heart procedures.
Additionally, in September 2021, Abbott announced the acquisition of Walk Vascular, LLC, a medical device company with a minimally invasive mechanical aspiration thrombectomy system designed to remove peripheral blood clots. Walk Vascular's peripheral thrombectomy systems will be incorporated into Abbott's existing endovascular product portfolio. The collaboration enabled Abbott to increase the range of its peripheral vascular services.
Therefore, owing to the above-mentioned factors, the growth of the studied market is anticipated in the North America Region.
The peripheral vascular devices market is slightly consolidated in nature due to the presence of a few companies operating globally as well as regionally. The competitive landscape includes an analysis of a few international as well as local companies which hold the market shares and are well known. Abbott Laboratories, Boston Scientific Corporation, Becton, Dickinson and Company, Cook, Medtronic, Cordis Corporation, Edward Lifesciences, and Volcano Corporation among others.