
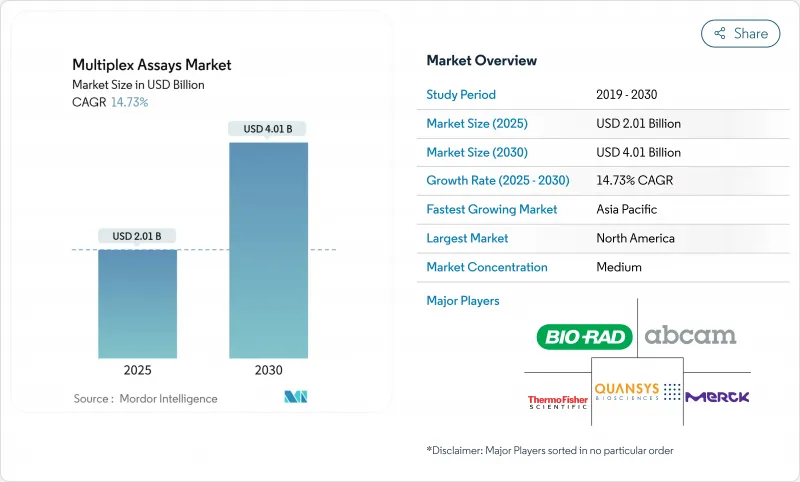
멀티플렉스 어세이 시장은 2025년에 20억 1,000만 달러로 추정되고, 2030년에는 40억 1,000만 달러에 이를 전망이며, CAGR 14.73%로 진전될 것으로 예측됩니다.

정밀의료에 대한 노력 증가, CRISPR을 이용한 진단의 비약적 진보, 40색 이상의 플로우 사이토메트리 플랫폼의 일상적 이용이 이 확대를 지지하고 있습니다. 임상 실험실이 멀티플렉스 포맷을 선호하는 이유는 샘플의 필요량을 줄이고, 납기를 단축하며, 비용을 관리하는 반면, 단일 플렉스 접근법으로는 대응할 수 없는 다중 파라미터에 대한 인사이트를 얻을 수 있기 때문입니다. 제약 스폰서는 바이오마커를 풍부하게 포함하는 시험 설계를 임상시험에 통합하고 있으며, 규제 당국은 멀티플렉스 검출에 의존하는 동반진단제를 그 어느 때보다 폭넓게 허가하고 있습니다. 동시에 병원은 침대 측에서 의사 결정을 개선하고 항균제의 오용을 억제하기 위해 호흡기와 패혈증의 검사에 신드로믹 패널을 채용하여 멀티플렉스 어세이 시장을 더욱 촉진하고 있습니다.
분자 특이적 치료에 대한 의존도가 증가함에 따라 여러 바이오마커를 한 번에 읽는 검사가 필요합니다. Thermofisher가 2024년 Olink를 31억 달러에 인수함에 따라 높은 처리량의 근접 확장 단백질 패널이 임상 워크플로우의 주류가 되었습니다. FDA는 500 유전자의 범종양 동반진단제인 TruSight Oncology Comprehensive를 승인하고, 데이터 밀도가 높은 프로파일링을 공식적으로 지지했습니다. 대규모 기준 실험실에서는 현재 루푸스의 진단을 정밀화하기 위해 자기 항체와 보체 마커를 결합하는 것이 일상적으로 이루어지고 있으며, 단일 분석보다 민감도가 향상되고 있음이 연구에 의해 나타났습니다. 또한 머신러닝 모델은 복잡한 서명을 명확한 지침으로 추출하여 해석력을 더욱 향상시킵니다.
인구의 고령화가 만성 질환의 모니터링을 촉진하는 반면, 재발하는 아웃 브레이크는 광범위한 병원체 패널에 대한 수요를 증가시키고 있습니다. 집중 치료 환경에서의 프로스펙티브 테스트는 호흡기 증후군 검사가 배양과 비교하여 적절한 치료 시간을 개선하는 것으로 확인되었습니다. FDA는 SARS-CoV-2, 인플루엔자 A/B, RSV의 4 병원체의 분자 검사를 20분 내에 실시하는 것을 허가하고, 신속한 다중 검출의 공중 보건 가치를 강조하고 있습니다. 만성 신장병 프로그램은 현재 유전체, 단백질체학 및 메타보로믹스를 하나의 분석에 통합하고 조기 악화를 국기함으로써 멀티오믹스와 예방 의료 간의 임상적 연결을 강화하고 있습니다. 팬데믹 후 임상가는 일상적인 감시 처리량을 유지하면서 새로운 위협에 신속하게 대응할 수 있는 패널을 기대하고 있으며 멀티플렉스 어세이 시장은 상승 기조를 유지하고 있습니다.
최상급의 스펙트럼 흐름 사이토미터는 50만 달러 이상이며, 이를 지원하는 인프라가 소유자의 부담을 늘리고 있습니다. QIAGEN은 2024년 팬데믹 후 수요 부진으로 자동 PCR 타워를 철수했는데, 이는 자본 예산이 박박한 경우의 상업적 위험을 보여줍니다. 소규모 시설에서는 품질 관리 및 데이터 처리에 관한 학습 곡선이 어려워 투자 회수가 지연됩니다.
2024년 멀티플렉스 어세이 시장 점유율에서는 단백질 패널이 41.76%를 차지했으며, 약물 표적의 검증 및 임상 의사 결정 지원의 지위를 확고히 하고 있습니다. 비드 기반 면역분석과 프록시미티 익스텐션 방법을 통해 마이크로리터 시료로부터 수백 유형의 사이토카인을 동시에 정량할 수 있습니다. 핵산 카테고리에서는 CRISPR과 차세대 시퀀싱이 종양학 뿐만 아니라 이식 모니터링 및 감염성 제노타이핑으로 확대됨에 따라 2030년까지 연평균 복합 성장률(CAGR)이 17.24%가 될 것으로 예상되고 있습니다. 세포 기반 포맷은 면역종양학 연구에 도움이 되며, 단백질과 유전자의 통합 워크플로우는 멀티오믹스의 미래를 말합니다.
통합 동향은 제품 설계를 재구성합니다. 2024년에 출시된 Thermofisher의 스텔라 질량 분석기는 10배의 정량 감도를 실현하여 실험실이 단백질체학 및 유전체학을 공유 자동화에서 결합할 수 있도록 합니다. 광범위한 유전체 동반진단제가 규제 당국으로부터 승인되면 대규모 멀티플렉스 시퀀싱의 효능이 입증되고 핵산 혁신에 대한 투자가 강화됩니다. 이러한 힘이 수렴함에 따라 멀티플렉스 어세이 시장은 분자 클래스의 다양화를 계속하고 있습니다.
유동세포계측법은 정착된 장비 그룹과 광범위한 임상 가이드라인에 따라, 2024년 매출액의 32.45%를 차지했습니다. 스펙트럼 언믹싱은 현재 채널당 더 많은 마커를 분해하고 플랫폼의 수명을 연장하고 있습니다. 그러나 질량세포계수는 CAGR 15.23%를 보일 것으로 예측되며, 메탈태그 항체를 활용하여 스펙트럼 중복 없이 100개 이상의 파라미터를 읽어냅니다. Standard BioTools의 CyTOF XT PRO는 이 루틴 테스트 워크플로우에 대한 추진을 보여줍니다.
실시간 PCR은 호흡기 및 패혈증 패널에 필수적이며, 시퀀싱 플랫폼은 종양 및 희귀질환의 검사에 적합합니다. Luminex xMAP는 높은 처리량의 단백질 요구를 충족시키고, 마이크로어레이는 틈새 유전자 발현 애플리케이션을 차지합니다. AI 툴이 패널 디자인과 자동 게이팅을 용이하게 하고 임상 실험실에서 고급 유동 세포 계측법 및 대량 세포 계측법의 광범위한 채택을 촉진함에 따라 경쟁과의 차이가 급속히 줄어들고 있습니다.
북미는 2024년 매출의 40.56%를 차지했는데, 이는 기술의 조기 도입 및 명확한 상환 경로 때문입니다. 서모피셔는 2029년까지 미국에서 제조 및 연구개발을 확대하기 위해 20억 달러를 기부할 것을 표명하고 이 지역의 리더십이 강화되었습니다. 유럽은 성능 주장을 표준화하는 IVDR의 의무화에 이끌려 이어지지만, 적합성 평가의 병목에 의해 일부 출시가 지연되고 있습니다.
아시아태평양은 중국, 일본, 한국이 바이오파마 투자를 촉진하고 분자 프로파일링에 대한 상환 코드를 갱신하기 때문에 CAGR 16.89%의 기세가 전망됩니다. 시약이나 기기의 현지 생산에 정부 보조금이 채워져 수입 의존도가 저하되고 있습니다. 인도의 병원 체인은 사망률 감소를 위해 멀티플렉스 패혈증 패널을 채택하여 신흥 시장의 잠재력을 보여줍니다.
중동 및 아프리카에서는 3차 의료 센터에서 신드로믹 호흡기 패널의 시험적 전개를 볼 수 있으며, 종종 국제적인 지원에 의해 지원되고 있습니다. 남미에서는 브라질의 주요 암 전문 병원이 면역 요법 모니터링을 위해 대량 세포 계측기를 도입하는 등 선택적인 성장을 볼 수 있습니다. 각 지역에서 규제 시계 및 인프라의 성숙도가 다르기 때문에 멀티플렉스 어세이 업체 시장 진입 계획은 개별적으로 설정됩니다.
The multiplex assays market is valued at USD 2.01 billion in 2025 and is projected to reach USD 4.01 billion by 2030, advancing at a 14.73% CAGR.
A growing commitment to precision medicine, breakthroughs in CRISPR-enabled diagnostics, and routine use of 40-plus-color flow cytometry platforms underpin this expansion. Clinical laboratories favor multiplex formats because they reduce sample requirements, shorten turnaround time, and control costs while delivering multi-parameter insights that singleplex approaches cannot match. Pharmaceutical sponsors are embedding biomarker-rich study designs into trials, and regulatory authorities are clearing ever-broader companion diagnostics that rely on multiplex detection. At the same time, hospitals are adopting syndromic panels for respiratory and sepsis testing to improve bedside decision-making and curb antimicrobial misuse, further propelling the multiplex assays market.
Growing reliance on molecule-specific therapies demands tests that read several biomarkers at once. Thermo Fisher's USD 3.1 billion purchase of Olink in 2024 helped bring high-throughput proximity extension protein panels into mainstream clinical workflows. The FDA endorsed TruSight Oncology Comprehensive, a 500-gene pan-tumor companion diagnostic, illustrating official support for data-dense profiling. Large reference labs now routinely combine autoantibody and complement markers to refine lupus diagnoses, studies show improved sensitivity over single analytes. Health systems recognize that multiplex strategies cut repeat testing costs and speed treatment alignment, and machine-learning models further boost interpretive power by distilling complex signatures into clear guidance.
Aging populations drive chronic disease monitoring while recurrent outbreaks reinforce demand for broad pathogen panels. Prospective trials in intensive care settings confirm that respiratory syndromic tests improve time-to-appropriate therapy versus cultures. The FDA authorized a 20-minute four-pathogen molecular test for SARS-CoV-2, Influenza A/B, and RSV, underscoring the public-health value of rapid multiplex detection. Chronic kidney disease programs now integrate genomics, proteomics, and metabolomics in one assay to flag early deterioration, tightening the clinical link between multiomics and preventive care. Post-pandemic, clinicians expect panels that pivot quickly to new threats while maintaining throughput for routine surveillance, keeping the multiplex assays market on an upward trajectory.
A top-tier spectral flow cytometer costs more than USD 500,000, and supporting infrastructure adds to ownership burden. QIAGEN withdrew its automated PCR tower in 2024 due to shallow post-pandemic demand, illustrating commercial risk when capital budgets tighten. Smaller sites face steep learning curves around quality control and data handling, delaying return on investment.
Other drivers and restraints analyzed in the detailed report include:
For complete list of drivers and restraints, kindly check the Table Of Contents.
Protein panels accounted for 41.76% of multiplex assays market share in 2024, cementing their status in drug-target validation and clinical decision support. Bead-based immunoassays and proximity extension methods enable simultaneous quantification of hundreds of cytokines from microliter samples. The nucleic-acid category is set to log a 17.24% CAGR to 2030 as CRISPR and next-generation sequencing expand beyond oncology into transplant monitoring and infectious-disease genotyping. Cell-based formats serve immuno-oncology research, while integrated protein-gene workflows herald a multiomics future.
Integration trends reshape product design. Thermo Fisher's Stellar mass spectrometer, introduced in 2024, delivers ten-fold better quantitative sensitivity, encouraging labs to pair proteomics with genomics on shared automation. Regulatory approval of broad genomic companion diagnostics validates large-scale multiplex sequencing, reinforcing investment in nucleic-acid innovation. As these forces converge, the multiplex assays market continues to diversify across molecular classes.
Flow cytometry delivered 32.45% of 2024 revenue, thanks to entrenched instrument fleets and extensive clinical guidelines. Spectral unmixing now resolves more markers per channel, extending platform life. Mass cytometry, however, is forecast to grow at 15.23% CAGR, leveraging metal-tagged antibodies to read over 100 parameters without spectral overlap. Standard BioTools' CyTOF XT PRO exemplifies this push toward routine trial workflows.
Real-time PCR remains vital for respiratory and sepsis panels, whereas sequencing platforms capture oncology and rare-disease testing. Luminex xMAP satisfies high-throughput protein needs, and microarrays occupy niche gene-expression applications. Competitive gaps close rapidly as AI tools ease panel design and auto-gating, encouraging broader adoption of advanced flow and mass cytometry in clinical labs.
The Multiplex Assays Market Report is Segmented by Type (Cell-Based Multiplex Assays, Protein Multiplex Assays, and More), Technology (Multiplex Real-Time PCR, Flow Cytometry, and More), Application (Infectious Disease Diagnostics, Oncology & Companion Diagnostics and More), End-User (Pharmaceutical & Biopharmaceutical Companies, and More), and Geography. The Market Forecasts are Provided in Terms of Value (USD).
North America captured 40.56% of 2024 revenue owing to early technology adoption and clear reimbursement paths. Thermo Fisher pledged USD 2 billion to expand U.S. manufacturing and R&D through 2029, reinforcing the region's leadership. Europe follows, guided by IVDR mandates that standardize performance claims, though conformity-assessment bottlenecks slow some launches.
Asia-Pacific is poised for a 16.89% CAGR as China, Japan, and South Korea boost biopharma investment and update reimbursement codes for molecular profiling. Government grants fund local manufacturing of reagents and instruments, cutting import dependency. India's hospital chains adopt multiplex sepsis panels to reduce mortality, illustrating emerging-market potential.
The Middle East and Africa region sees pilot rollouts of syndromic respiratory panels in tertiary centers, often backed by international aid. South America shows selective growth, with Brazil's leading oncology hospitals installing mass cytometers for immunotherapy monitoring. Across regions, differing regulatory clocks and infrastructure maturity dictate tailored go-to-market plans for multiplex assay vendors.