
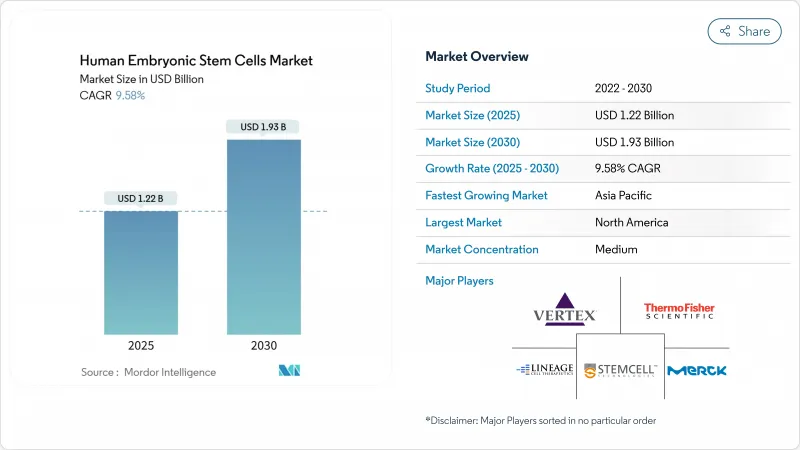
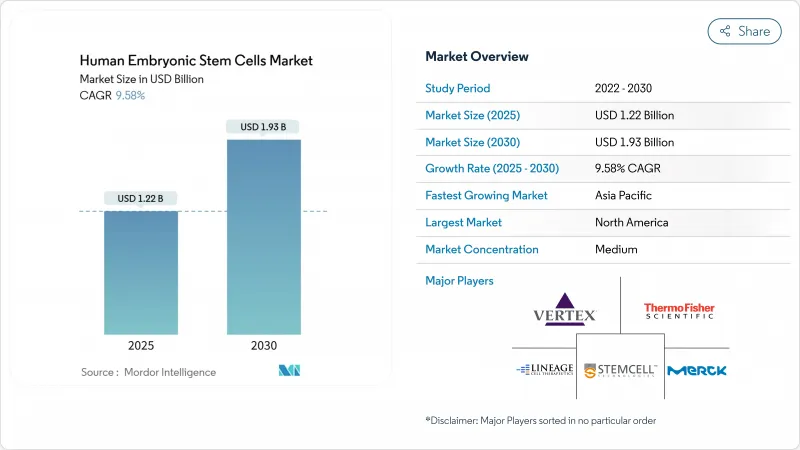
인간 배아 줄기세포 시장 규모는 2025년에 12억 2,000만 달러로 추정되고, 예측 기간(2025-2030년) 중 CAGR 9.58%로 성장할 전망이며, 2030년에는 19억 3,000만 달러에 달할 것으로 예측됩니다.
CRISPR을 이용한 라인 엔지니어링의 진보, 윤리적으로 이용 가능한 잉여 체외수정(IVF) 배아의 꾸준한 증가, 제노프리 GMP 배양 시스템의 상업적 이용 가능성은 심장, 망막, 내분비 질환에 걸친 치료의 가능성을 확대합니다. 자동화된 폐쇄 시스템 바이오프로세싱은 오염 위험을 줄이고 배치 처리 능력을 두 배로 증가시키며 인간 배아 줄기세포 시장의 조기 이동 경쟁력을 갈수록 산업계로의 침투를 증가시킵니다. FDA의 RMAT 패스웨이와 일본의 패스트트랙 승인으로 대표되는 규제 당국의 지원은 임상에서 시장으로의 타임라인을 앞당겨 수십억 달러의 자금 제공을 약속시킵니다. 한편, 유전자 편집의 개척자와 제조 전문가의 학제 간 협업은 시장 개척 주기를 단축하고 인간 배아 줄기세포 시장에서 지적 재산의 수비 범위를 넓힙니다. 윤리적 액티비즘 증가 및 비용 상승 압력은 여전히 주시점이지만, 기술 주도의 생산성 향상은 당분간의 역풍을 상쇄하는 기세입니다.
심혈관계 질환과 암은 세계적으로 사망률의 대부분을 차지하고 있으며, 다계통의 복구 솔루션에 대한 지속적인 임상 수요를 창출하고 있습니다. 인간 배아 줄기세포에서 유래된 심근 스페로이드는 돼지의 경색 모델에서 수축력을 회복시키고 현재 인간 최초의 허혈성 심근증 임상시험에 들어가 있습니다. 인구 수준에서 심장 치료에 대한 지출이 3,500억 달러를 넘어가면서 상업적 관심이 증가하고 있으며, 심장 대사 프로그램은 인간 배아 줄기세포 시장에서 주요 가치 촉진요인이 되고 있습니다. hESC를 파종한 3D 프린팅 심근 스캐폴드와 같은 부가제조의 돌파구는 트랜스레이셔널 패스웨이를 더욱 단축합니다. 이러한 데이터를 종합하면 질병 수식의 가능성을 검증하고 2030년까지 프리미엄 가격을 설정할 수 있는 기회를 강화합니다.
미국에서는 1,200개 이상의 세포 및 유전자 치료의 임상시험이 진행되고 있어 재생의료가 프린지 치료에서 최전선의 치료로 어떻게 이행하고 있는 지 나타내고 있습니다. 인간 배아 줄기세포에 의존하는 동종요법 플랫폼은 기성품 투여를 가능하게 하고, 자가 치료의 역사적인 규모의 한계를 해결합니다. 일본의 60개가 넘는 iPS 세포 연구는 일관된 정책, 상환의 명확화, 제조 인센티브가 채택을 가속화하는 방법을 보여줍니다. FDA에 의한 간엽계 간질세포 치료의 획기적인 승인은 임상적으로 검증된 제품을 규제 당국이 승인할 준비가 있음을 나타내며, 간접적으로 hESC 개발자에게 이익을 가져옵니다. hESC 유래 각막 상피를 이용한 시력 회복의 성공(90% 이상의 효능)은 사회적 신용을 높이고 환자 모집과 투자자에게 자금 유입에 긍정적인 피드백 루프를 촉진합니다.
현재의 hESC 기반 치료법은 클린룸에서의 수작업과 낮은 수율로 인해 1회 투여당 10만 달러를 초과하는 경우가 많습니다. AI가 유도하는 로봇 공학은 노동 투입을 50% 삭감하고, 로트 간의 일관성을 실현하며, 5년 이내에 5만 달러 이하로 손익 분기점의 제조가 가능하게 됩니다. 뉴저지에 있는 서모 피셔의 4억 7,500만 달러 CDMO 사이트는 비용 절감과 규제 준수를 목표로 하는 업계 규모의 투자 예입니다. 모듈식 로봇 클러스터는 확장, 수확 및 최종 채우기 마무리를 재현성있게 처리하여 캠페인 시간을 단축하고 배치 불량 위험을 줄입니다. 노스 이스턴 대학의 예측 AI 모델은 영양 공급과 통과 타이밍을 더욱 최적화하고 수율을 산업용 벤치마크로 밀어 올립니다. 지불자의 감시와 가격에 민감한 신흥 시장 수요를 상쇄하기 위해서는 이러한 이익을 지속시켜야 합니다.
재생 의료는 2024년 인간 배아 줄기세포 시장 점유율의 58.57%를 차지하였고, 지리적 위축증 환자에서 OpRegen의 +5.5 문자 시력 향상 등의 임상 결과에 의해 뒷받침되었습니다. 척수 복구, 췌도 대체, 심근 재근육화는 현재 다시설 임상시험의 선두에서 이 부문의 압도적인 수익 궤도를 강화하고 있습니다. RMAT 및 선구의 지정을 받는 프로그램이 늘어남에 따라, 지불자는 상환을 정당화하는 실제 임상 증거를 획득하고, 인간 배아 줄기세포 시장의 선순환에 박차를 가하고 있습니다.
줄기세포 생물학 조사는 2030년까지 연평균 복합 성장률(CAGR) 10.86%로 성장을 지속할 전망이며, 자동화된 유전자 편집 스크리닝과 인간 조직의 복잡성을 재현할 수 있는 오가노이드 플랫폼으로부터 혜택을 받습니다. CRISPR을 이용한 계통 추적과 하이콘텐트 페노믹스는 표적 동정의 타임라인을 단축하고, 새로운 오가노이드 공배양 시스템은 질환 모델의 정밀도를 5배로 높입니다. 아카데믹한 핵심 시설이 피포 서비스 모델로 전환됨에 따라, 연구비는 시약 및 라이선싱 로열티로 환원되어 공급망 참가자의 경상 수익 풀이 확대됩니다. 검색 용도의 인간 배아 줄기세포 시장 규모는 멀티플렉스 스크린이 정밀의료 파이프라인에 필수적이 됨에 따라 꾸준히 상승할 것으로 예측됩니다.
북미는 NIH와 CIRM의 왕성한 자금 제공, RMAT에 의한 신속한 심사, CDMO의 광범위한 능력을 배경으로 2024년 세계 매출의 42.16%를 유지했습니다. 스탠포드 주도 심장 시험, 노스웨스턴 척수 이니셔티브, Thermo Fisher의 프린스턴 신거점은 이 지역의 실험실에서 출시까지의 통합을 총체적으로 보여줍니다. 배아 연구에 대한 연방정부의 자금 제공을 둘러싼 정치적 불확실성은 전략적 리스크가 되지만, 다양한 민간투자가 잠재적인 공적 예산의 변동을 완화하고 있습니다.
아시아태평양은 가장 급성장하는 클러스터로 일본의 조건부 승인 제도와 중국의 국영 리서치 파크를 배경으로 2030년까지 연평균 복합 성장률(CAGR)이 11.41%로 진행될 전망니다. 일본에서는 60개 이상의 임상시험이 활발히 이루어지고 있으며, 규제당국의 기민성을 부각하고 있는 한편, 스미토모제약과 니콘 론자의 제조 제휴는 다국적 파트너로부터의 자본 유입을 실증하고 있습니다. 정부 보조금은 시설 건설과 노동자 훈련에 충당되어 인간 배아 줄기세포 시장에서 현지 공급망의 성숙도를 높입니다.
유럽 전망은 2027년 SOHO 규정의 효과적인 개발에 달려 있습니다. 독일과 영국은 주요 학술 클러스터를 유지하고 있습니다. 영국의 인공 배아에 관한 실시 규범은 대륙의 표준을 형성할 수 있는 정책 혁신의 신호입니다. 프랑스와 이탈리아는 안과 및 연골 복구의 틈새 분야에 주력하고 있으며, 스칸디나비아의 컨소시엄은 환자 접근을 넓히기 위해 저온 물류에 투자하고 있습니다. 서유럽에서는 상환 장애물이 여전히 높지만, 국경을 넘은 협력 체제와 EU 전체의 HTA 개혁에 의해 인증 제품 시장 접근이 합리화될 것으로 기대됩니다.
The Human Embryonic Stem Cells Market size is estimated at USD 1.22 billion in 2025, and is expected to reach USD 1.93 billion by 2030, at a CAGR of 9.58% during the forecast period (2025-2030).
Advances in CRISPR-enabled line engineering, a steadily growing pool of ethically sourced surplus IVF embryos, and the commercial availability of xeno-free GMP culture systems collectively expand therapeutic horizons across cardiac, retinal, and endocrine disorders. Industrial uptake rises as automated, closed-system bioprocessing cuts contamination risk and doubles batch throughput, sharpening the competitive edge for early movers in the human embryonic stem cells market. Regulatory support, exemplified by the FDA's RMAT pathway and Japan's fast-track approvals, accelerates clinic-to-market timelines and attracts multi-billion-dollar funding commitments. Meanwhile, cross-disciplinary collaborations between gene-editing pioneers and manufacturing specialists compress development cycles and broaden intellectual-property defensibility within the human embryonic stem cells market. Heightened ethical activism and rising cost pressures remain watchpoints, but technology-driven productivity gains are on track to offset near-term headwinds.
Cardiovascular disorders and cancer together account for the majority of mortality worldwide, creating sustained clinical demand for multi-lineage repair solutions. Human embryonic stem-cell-derived cardiac spheroids restore contractility in porcine infarct models and are now entering first-in-human ischemic cardiomyopathy trials. hESC-derived islet cells (VX-880) achieved insulin independence in 10 of 12 type 1 diabetes patients, underscoring the breadth of application potential. Commercial interest intensifies as population-level spending on cardiac care exceeds USD 350 billion, positioning cardiometabolic programs as headline value drivers within the human embryonic stem cells market. Additive-manufacturing breakthroughs, such as 3-D-printed myocardium scaffolds seeded with hESCs, further shorten translational pathways. Collectively, these data validate disease-modifying potential and reinforce premium pricing opportunities through 2030.
More than 1,200 active cell and gene therapy trials in the United States showcase how regenerative medicine is moving from fringe to frontline care. Allogeneic platforms that rely on human embryonic stem cells enable off-the-shelf dosing, solving historical scale limitations of autologous procedures. Japan's 60-plus iPS cell studies illustrate how cohesive policy, reimbursement clarity, and manufacturing incentives foster accelerated adoption. The FDA's landmark approval of a mesenchymal stromal cell therapy signals regulator readiness to clear clinically validated products, indirectly benefiting hESC developers. Vision-restoration successes using hESC-derived corneal epithelium (>=90% efficacy) elevate public trust, fueling positive feedback loops for patient recruitment and investor inflows.
Current hESC-based therapies often exceed USD 100,000 per dose, driven by manual clean-room operations and low process yields. AI-guided robotics slash labor inputs by 50% and deliver lot-to-lot consistency, pointing to breakeven manufacturing at sub-USD 50,000 within five years. Thermo Fisher's USD 475 million CDMO site in New Jersey exemplifies industry-scale investment targeting cost compression and regulatory compliance. Modular robotic clusters reproducibly handle expansion, harvesting, and final fill-finish, shortening campaign times and reducing batch failure risk. Predictive AI models from Northeastern University further optimize nutrient feeds and passage timing, pushing yields toward industrial benchmarks. These gains must sustain momentum to offset payer scrutiny and price-sensitive emerging-market demand.
Other drivers and restraints analyzed in the detailed report include:
For complete list of drivers and restraints, kindly check the Table Of Contents.
Regenerative medicine captured 58.57% of the human embryonic stem cells market share in 2024, buoyed by clinical readouts such as +5.5-letter visual-acuity gains for OpRegen in geographic atrophy patients. Spinal cord repair, pancreatic islet replacement, and cardiac remuscularization now headline multi-center trials, reinforcing the segment's dominant revenue trajectory. As more programs secure RMAT or Sakigake designations, payers gain real-world evidence to justify reimbursement, fueling a virtuous adoption cycle within the human embryonic stem cells market.
Stem-cell biology research, registering a 10.86% CAGR to 2030, benefits from automated genome-editing screens and organoid platforms capable of recapitulating human tissue complexity. CRISPR-powered lineage-tracing and high-content phenomics shorten target-identification timelines, while novel organoid co-culture systems elevate disease-model accuracy five-fold. As academic core facilities transition to fee-for-service models, research spending funnels back into reagents and line-licensing royalties, enlarging recurring-revenue pools for supply-chain participants. The human embryonic stem cells market size for discovery applications is projected to climb steadily as multiplex screens become integral to precision-medicine pipelines.
The Human Embryonic Stem Cells Market Report is Segmented by Application (Regenerative Medicine, Stem-Cell Biology Research, Tissue Engineering, Toxicology Testing), Product Type (hESC Lines, Culture Media & Reagents, Instruments & Consumables), and Geography (North America, Europe, Asia-Pacific, Middle East and Africa, South America). The Market Forecasts are Provided in Terms of Value (USD).
North America retained 42.16% of global revenue in 2024, anchored by robust NIH and CIRM funding, RMAT-enabled fast-track reviews, and extensive CDMO capacity. Stanford-led cardiac trials, Northwestern's spinal cord initiatives, and Thermo Fisher's new Princeton site collectively exhibit the region's lab-to-launch integration. Political uncertainty around federal funding for embryonic research poses a strategic risk, but diversified private investment cushions potential public-budget fluctuations.
Asia-Pacific is the fastest-growing cluster, advancing at an 11.41% CAGR through 2030 on the back of Japan's conditional-approval regime and China's state-backed research parks. Over 60 active Japanese clinical trials highlight regulatory agility, while Sumitomo Pharma and Nikon-Lonza manufacturing alliances demonstrate capital inflows from multinational partners. Government grants cover facility build-outs and workforce training, amplifying local supply-chain maturity within the human embryonic stem cells market.
Europe's outlook hinges on effective rollout of the SoHO regulation in 2027. Germany and the United Kingdom maintain leading academic clusters; the UK's code of practice for synthetic embryos signals policy innovation that may shape continental standards. France and Italy focus on ophthalmology and cartilage repair niches, while Scandinavian consortia invest in cryogenic logistics to widen patient access. Western European reimbursement hurdles persist, but cross-border collaboration and EU-wide HTA reforms are expected to streamline market access for certified products.