
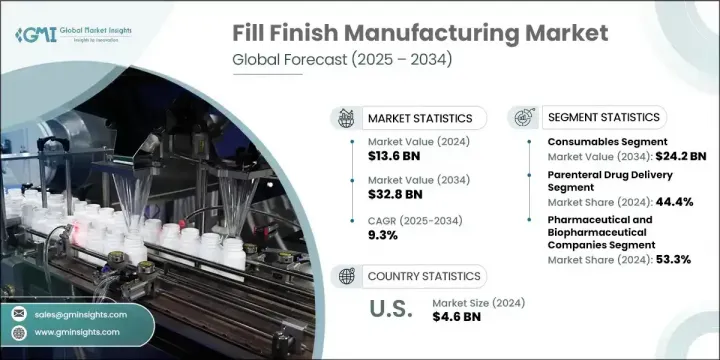
세계의 Fill-Finish 제조 시장 규모는 2024년에는 136억 달러로 평가되었고, CAGR 9.3%를 나타내 2034년에는 328억 달러에 이를 것으로 추정됩니다.
시장 성장의 원동력은 제조 기술의 지속적인 진화, 무균 약물 전달 형식에 대한 수요 증가, 바이오 의약품 개발의 대폭적인 확대입니다. Fill-Finish 제조는 비경구 의약품의 무균성, 정확성 및 안전한 포장을 보장하는 데 중요한 역할을 합니다. 백신, 생물학적 제제 및 기타 주사 제제를 프리필드 주사기, 바이알, 앰플, 카트리지 등의 포맷에 무균 충전합니다.

프리필드 주사기의 인기가 증가함에 따라, 특히 복잡한 생물학적 제제의 경우, 오염 및 투여 실수를 최소화하는 능력에 의해 이 시장을 재형성하고 있습니다. 제약 기업은 수요 증가에 대응하고 진화하는 규제 프레임워크을 준수하기 위해 고효율 충전 시스템, 첨단 무균 패키징 및 디지털 품질 관리 도구에 대한 투자를 늘리고 있습니다. 주사용 생물 제제의 승인과 RTU(Ready-To-Use) 용기 시스템의 상승이 결합되어 정밀 공학을 기반으로 하는 충전 마감 솔루션에 대한 수요가 더욱 높아지고 있습니다.
| 시장 범위 | |
|---|---|
| 시작 연도 | 2024년 |
| 예측 연도 | 2025-2034년 |
| 시작 금액 | 136억 달러 |
| 예측 금액 | 328억 달러 |
| CAGR | 9.3% |
2024년 소모품 부문은 102억 달러를 창출했고 2034년에는 242억 달러에 이를 것으로 전망됩니다. 카트리지, 바이알, 프리필드 주사기와 같은 소모품은 주사제의 제형화 및 전달에 널리 사용됩니다. 이 품목은 무균 상태를 유지하고 정확한 복용량을 제공하며 오염 위험을 줄이는 데 필수적입니다. 대량으로 소비되고, 1회 소진이라는 성질로부터, 최신의 의약품 제조 환경에서는 충전 마무리 공정의 필수적인 구성 요소가 되고 있습니다. 특히 절대적인 정밀도와 오염 제어가 가장 중요한 생물 제제와 백신 제조에서의 채용률이 높습니다.
제약·바이오 제약 기업 부문은 2024년에 53.3%의 점유율을 차지했습니다. 이 부문은 무균 주사제의 상업적 규모의 생산 및 포장에 크게 관여하기 때문에 계속 이어지고 있습니다. 이 회사는 고급 충전 라인, 폐쇄 시스템 격리 장비 및 지능형 로봇에 의존하며 전체 제품 파이프라인의 정확성, 무균성 및 규정 준수를 보장합니다. 개인화된 치료제 및 단일클론 항체를 포함한 의약품 개발 파이프라인의 확장은 복잡한 제품 특성 및 맞춤형 포맷을 수용할 수 있는 고도로 전문화된 충전 마감 장비 및 소모품의 요구를 증가시키고 있습니다.
북미의 Fill-Finish 제조 점유율은 2024년에 37%가 되었습니다. 이 지역은 의약품 인프라가 고도로 발달하고 있으며 제조 수탁기관의 존재감이 강한 것이 지속적인 성장에 기여하고 있습니다. 북미의 규제 상황은 안전과 품질을 중시하면서 혁신을 지원합니다. 북미 제조업체는 생산량 증가에 대응하고 새로운 생물학적 제형에 적응하기 위해 자동화 기술과 무균 처리 기능을 빠르게 통합하고 있습니다. 첨단 패키징 시스템과 무균 소모품을 이용할 수 있는 것도 이 지역의 세계 생산고에 있어서의 우위성을 지지하고 있습니다.
Fill-Finish 제조 시장에서 사업을 전개하고 있는 유명한 기업은 Gerresheimer, Corning Incorporated, AST, SGD Pharma, SCHOTT Pharma, Stevanato Group, iMA Industria Macchine Automatiche, Steriline, Becton, Dickinson and Company, Bausch Strobel, Nipro, Groninger Kish 다라 등이 있습니다. 이러한 기업들은 장비에서 소모품에 이르기까지 시장의 다양한 부문에 걸쳐 강력한 비계를 유지하고 있습니다. Fill-Finish 제조 시장에서 활동하는 기업은 차세대 자동화, 로봇 공학, 모듈식 생산 라인에 적극적으로 투자하여 운영 유연성을 향상시키고 처리량을 높이고 있습니다. 바이오 의약품 개발 기업과의 전략적 제휴는 컨테이너 형식의 공동 개발과 제품 개발 기간의 단축에 도움이 됩니다. 규제 준수와 품질 보증에 대한 강한 관심은 클린 룸 기술과 무균 아이솔레이터에 대한 지속적인 투자를 촉진합니다. 대기업은 또한 지역별 수요에 대응하고 리드 타임을 단축하기 위해 세계적인 제조 거점을 확대하고 있습니다. 개인화 의약품 및 소량 생산에 대한 수요 증가에 대응하기 위해 여러 기업이 멀티 포맷 머신과 유연한 충전 라인으로 기술 혁신을 진행하고 있습니다. 또한, 공정의 신뢰성을 향상시키면서 충전 마무리의 복잡성을 줄이는 RTU 패키징 구성 요소의 개발에 연구개발의 강화가 쏟아지고 있습니다.
The Global Fill Finish Manufacturing Market was valued at USD 13.6 billion in 2024 and is estimated to grow at a CAGR of 9.3% to reach USD 32.8 billion by 2034. Market growth is being powered by the continued evolution of manufacturing technologies, increasing demand for sterile drug delivery formats, and a significant expansion in biopharmaceutical development. Fill finish manufacturing plays a vital role in ensuring the sterility, precision, and safe packaging of parenteral drug products. It involves the aseptic filling of vaccines, biologics, and other injectable formulations into formats like prefilled syringes, vials, ampoules, and cartridges.
The growing popularity of prefilled syringes is reshaping this market, driven by their ability to minimize contamination and dosage errors, particularly in the case of complex biologics. Pharmaceutical firms are increasing investment in high-efficiency filling systems, advanced sterile packaging, and digital quality control tools to meet rising demand and comply with evolving regulatory frameworks. The combination of injectable biologic approvals and the rise of Ready-To-Use (RTU) container systems is further enhancing the demand for precision-engineered fill finish solutions.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $13.6 Billion |
| Forecast Value | $32.8 Billion |
| CAGR | 9.3% |
In 2024, the consumables segment generated USD 10.2 billion and is projected to hit USD 24.2 billion by 2034. Consumables such as cartridges, vials, and prefilled syringes are used extensively in the formulation and delivery of injectable medications. These items are indispensable in preserving sterility, delivering accurate doses, and reducing the risk of contamination. Their high-volume consumption and single-use nature make them an integral component of the fill finish process in modern pharmaceutical manufacturing environments. Their adoption is especially high in biologics and vaccine production, where absolute precision and contamination control are paramount.
The pharmaceutical and biopharmaceutical companies segment held a 53.3% share in 2024. This segment continues to lead due to its substantial involvement in the commercial-scale production and packaging of sterile injectable drugs. These companies rely on advanced filling lines, closed-system isolators, and intelligent robotics to ensure precision, sterility, and regulatory compliance across their product pipelines. Their expansive drug development pipelines, including personalized therapies and monoclonal antibodies, are driving the need for highly specialized fill finish equipment and consumables that can handle complex product characteristics and customized formats.
North America Fill Finish Manufacturing Market held a 37% share in 2024. The region's highly developed pharmaceutical infrastructure and the strong presence of contract manufacturing organizations contribute to sustained growth. Its regulatory landscape supports innovation while emphasizing safety and quality. North American manufacturers are rapidly integrating automated technologies and aseptic processing capabilities to meet rising production volumes and adapt to newer biologic formulations. The availability of advanced packaging systems and sterile consumables further supports the region's dominance in global production output.
Prominent companies operating in the Fill Finish Manufacturing Market include Gerresheimer, Corning Incorporated, AST, SGD Pharma, SCHOTT Pharma, Stevanato Group, I.M.A. Industria Macchine Automatiche, Steriline, Becton, Dickinson and Company, Bausch+Strobel, Nipro, Groninger, Kishore Group, Borosil Scientific, and Maquinaria Industrial Dara. These firms continue to hold a strong foothold across various segments of the market, from equipment to consumables. Companies active in the fill finish manufacturing market are investing aggressively in next-gen automation, robotics, and modular production lines to improve operational flexibility and increase throughput. Strategic collaborations with biopharmaceutical developers help these players co-develop container formats and reduce product development timelines. A strong focus on regulatory compliance and quality assurance drives ongoing investments in cleanroom technology and aseptic isolators. Major players are also expanding their global manufacturing footprint to serve region-specific demand and reduce lead times. To cater to the rising demand for personalized drugs and small-batch production, several firms are innovating with multi-format machines and flexible filling lines. In addition, enhanced R&D efforts are being channeled into developing RTU packaging components that reduce fill finish complexities while improving process reliability.