
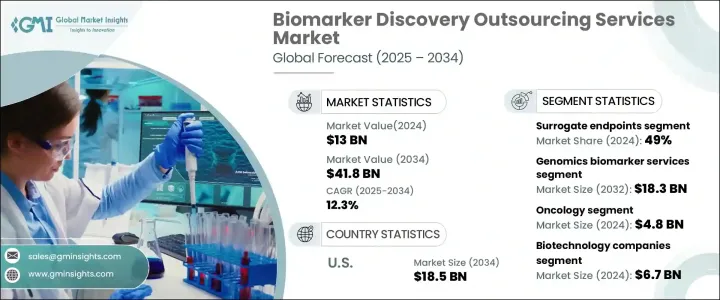
세계 바이오마커 탐색 아웃소싱 서비스 시장은 2024년 130억 달러로 평가되었고, 2025-2034년 연평균 12.3%의 성장률을 보일 것으로 예측됩니다.
이 시장은 만성질환 증가, 오믹스 기술의 급속한 발전, 제약 및 바이오테크놀러지 부문의 연구개발 투자 증가 등을 배경으로 크게 성장하고 있습니다. 개인 맞춤형 의료에 대한 관심이 높아지면서 암, 심혈관 질환, 당뇨병 등 만성질환의 조기 발견, 진단, 모니터링을 가능하게 하는 신규 바이오마커에 대한 수요가 더욱 증가하고 있습니다. 전 세계적으로 만성질환의 유병률이 증가함에 따라 표적 치료 전략을 촉진하는 바이오마커에 대한 수요는 지속적으로 증가하고 있습니다.

바이오마커 검증에 대한 정부 이니셔티브, 제약사와 연구기관의 협력, 규제 당국의 지원으로 시장 확대가 가속화되고 있습니다. 바이오마커 탐색에 인공지능(AI)과 머신러닝(ML)의 도입이 증가하면서 잠재적인 바이오마커를 신속하게 식별하고 검증할 수 있게 되면서 업계가 변화하고 있습니다. 신흥국은 헬스케어 지출 증가와 첨단 임상 연구센터의 설립으로 바이오마커 연구의 주요 기업로 부상하고 있습니다. 또한, 디지털 헬스 솔루션과 바이오인포매틱스 툴의 도입은 데이터 기반 바이오마커 탐색을 강화하여 환자 치료 결과를 개선하고 의약품 개발 프로세스를 효율화할 수 있도록 돕고 있습니다.
| 시장 범위 | |
|---|---|
| 시작 연도 | 2024년 |
| 예측 연도 | 2025-2034년 |
| 시작 금액 | 130억 달러 |
| 예상 금액 | 418억 달러 |
| CAGR | 12.3% |
이 시장은 예측, 예후, 안전성 바이오마커, 대체 엔드포인트, 기타 등 바이오마커 유형별로 분류되며, 2024년에는 대체 엔드포인트가 전체 매출의 49%를 차지하며 2025-2034년 12.1%의 연평균 복합 성장률(CAGR)을 나타낼 전망입니다. 성장할 것으로 예측됩니다. 대리 바이오마커는 연구자들이 치료 효과를 조기에 평가할 수 있게 함으로써 임상시험 과정을 크게 가속화하고, 임상시험에서 중요한 역할을 하고 있습니다. 특히 신흥 시장에서 널리 채택되고 있으며, 미국 FDA와 같은 당국이 암 및 희귀질환 치료제 승인에 대한 강력한 규제 지원을 제공함에 따라 시장 점유율이 지속적으로 상승하고 있습니다.
바이오마커 탐색 아웃소싱 서비스 시장은 유전체학, 단백질체학, 바이오인포매틱스, 기타 등 서비스 유형별로 세분화됩니다. 유전체학 및 바이오마커 서비스 분야에서만 2024년 57억 달러 규모 시장이 창출될 것으로 예상됩니다. 유전성 질환 환자 증가와 암, 신경퇴행성 질환과 같은 만성질환 관련 사망률 증가가 유전체 바이오마커 서비스에 대한 수요를 촉진하고 있습니다. 유전체 연구에 대한 공공 및 민간 부문의 투자 증가는 유전체 바이오마커의 발견과 검증을 촉진하고 시장을 더욱 강화시키고 있습니다. 차세대 염기서열 분석(NGS) 및 기타 고처리량 유전체 기술의 통합은 바이오마커 식별을 강화하여 보다 정확한 진단과 개인화된 치료 계획을 보장하고 있습니다.
미국의 바이오마커 발굴 아웃소싱 서비스 시장은 2034년까지 185억 달러에 달할 것으로 예상됩니다. 바이오테크놀러지 분야에서의 리더십, 첨단 의료 인프라, 확립된 규제 프레임워크가 이 분야에서 미국의 우위를 점하는 데 기여하고 있습니다. 미국 FDA는 바이오마커 검증을 위해 엄격하면서도 지원적인 규제 메커니즘을 도입하여 진단 및 의약품 개발 프로세스에 바이오마커를 성공적으로 통합할 수 있도록 지원하고 있습니다. 바이오 제약 기업, 학술 연구 기관, 정부 기관 간의 지속적인 파트너십은 기술 혁신과 시장 확대를 더욱 촉진하고 있습니다. 그 결과, 미국은 바이오마커 발굴 아웃소싱 서비스의 최전선에 있으며, 예측 기간 동안 세계 시장을 선도하고 있습니다.
The Global Biomarker Discovery Outsourcing Services Market was valued at USD 13 billion in 2024 and is projected to expand at a CAGR of 12.3% between 2025 and 2034. The market is witnessing significant growth, driven by the increasing incidence of chronic diseases, rapid advancements in omics technologies, and rising investments in research and development within the pharmaceutical and biotechnology sectors. The growing emphasis on personalized medicine is further fueling demand for novel biomarkers that enable early detection, diagnosis, and monitoring of chronic conditions such as cancer, cardiovascular diseases, and diabetes. With chronic disease prevalence on the rise worldwide, the demand for biomarkers to facilitate targeted treatment strategies continues to increase.
Rising government initiatives, collaborations between pharmaceutical companies and research organizations, and regulatory support for biomarker validation are accelerating market expansion. The increasing adoption of artificial intelligence (AI) and machine learning (ML) in biomarker discovery is also transforming the industry, allowing for faster identification and validation of potential biomarkers. Emerging economies are becoming key players in biomarker research due to rising healthcare expenditures and the establishment of advanced clinical research centers. Additionally, the adoption of digital health solutions and bioinformatics tools is enhancing data-driven biomarker discovery, leading to improved patient outcomes and more efficient drug development processes.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $13 Billion |
| Forecast Value | $41.8 Billion |
| CAGR | 12.3% |
The market is categorized into different biomarker types, including predictive, prognostic, safety biomarkers, surrogate endpoints, and others. Surrogate endpoints dominated the market in 2024, accounting for 49% of the total revenue share, and are expected to grow at a CAGR of 12.1% from 2025 to 2034. Surrogate biomarkers play a crucial role in clinical trials by enabling researchers to assess treatment efficacy early, significantly expediting the clinical study process. Their widespread adoption, particularly in emerging markets, and strong regulatory support from authorities such as the U.S. FDA for drug approvals in oncology and rare diseases continue to drive their market share.
The biomarker discovery outsourcing services market is further segmented by service type, which includes genomics, proteomics, bioinformatics, and others. The genomics biomarker services segment alone generated USD 5.7 billion in 2024. Increasing cases of genetic disorders and the rising mortality rates associated with chronic diseases like cancer and neurodegenerative conditions are fueling the demand for genomic biomarker services. Growing public and private sector investments in genomics research are expediting the discovery and validation of genomic biomarkers, further strengthening the market. The integration of next-generation sequencing (NGS) and other high-throughput genomic technologies is enhancing biomarker identification, ensuring more precise diagnostics and personalized treatment plans.
The U.S. Biomarker Discovery Outsourcing Services Market is expected to reach USD 18.5 billion by 2034. The country's leadership in the biotechnology sector, advanced healthcare infrastructure, and well-established regulatory framework are contributing to its dominance in this space. The U.S. FDA has implemented stringent yet supportive regulatory mechanisms for biomarker validation, ensuring the successful integration of biomarkers into diagnostics and drug development processes. Ongoing partnerships between biopharmaceutical companies, academic research institutions, and government agencies are further propelling innovation and market expansion. As a result, the U.S. remains at the forefront of biomarker discovery outsourcing services, leading the global market throughout the forecast period.